This report from the Guttmacher Institute estimates the cost to the Egyptian health system of treating cases of postpartum hemorrhage (PPH), which accounts for 20% of all maternal deaths in Egypt. Given the comparatively moderate amount of money spent on treating PPH and its persistence as a public health problem, the authors recommend that additional funds be allocated to its prevention and treatment.
The Cost to the Health System of Postpartum Hemorrhage in Egypt

Author(s)
Michael Vlassoff, H. A. Abdalla and Vivian GorReproductive rights are under attack. Will you help us fight back with facts?
Key Points
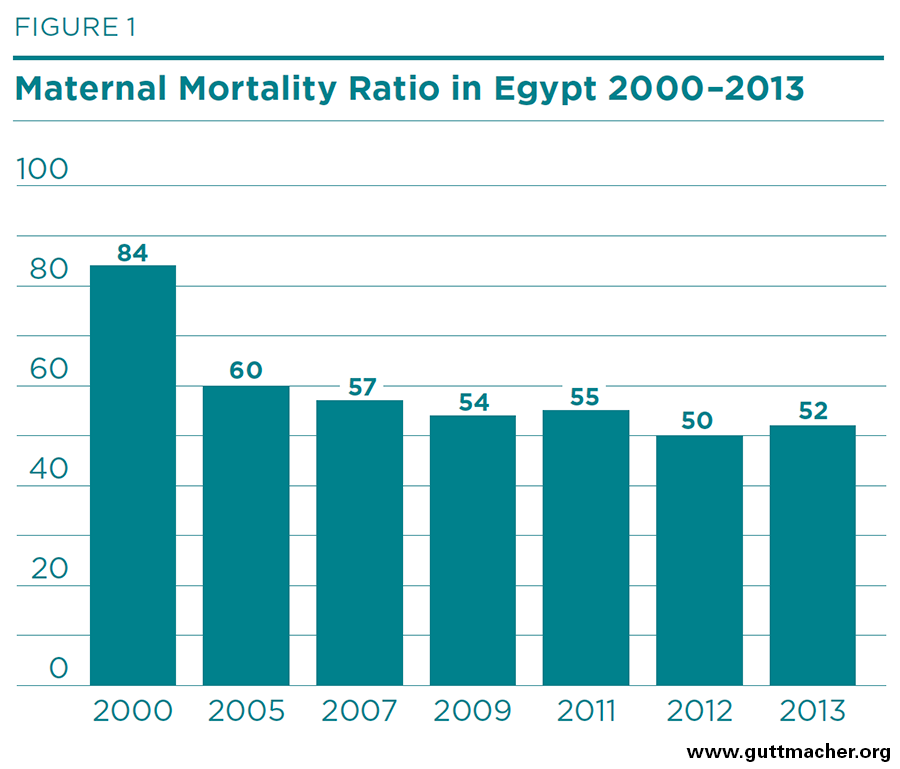
- The maternal mortality ratio in Egypt declined from 84 deaths per 100,000 live births in 2000 to 54 deaths in 2009, but has plateaued since then. About 20% of maternal deaths nationwide are due to postpartum hemorrhage (PPH).
- This report describes the results of a study on the cost of treating PPH in two district hospitals located in El Beheira Governorate. The study was conducted during 2013–2014 and used a Delphi approach.
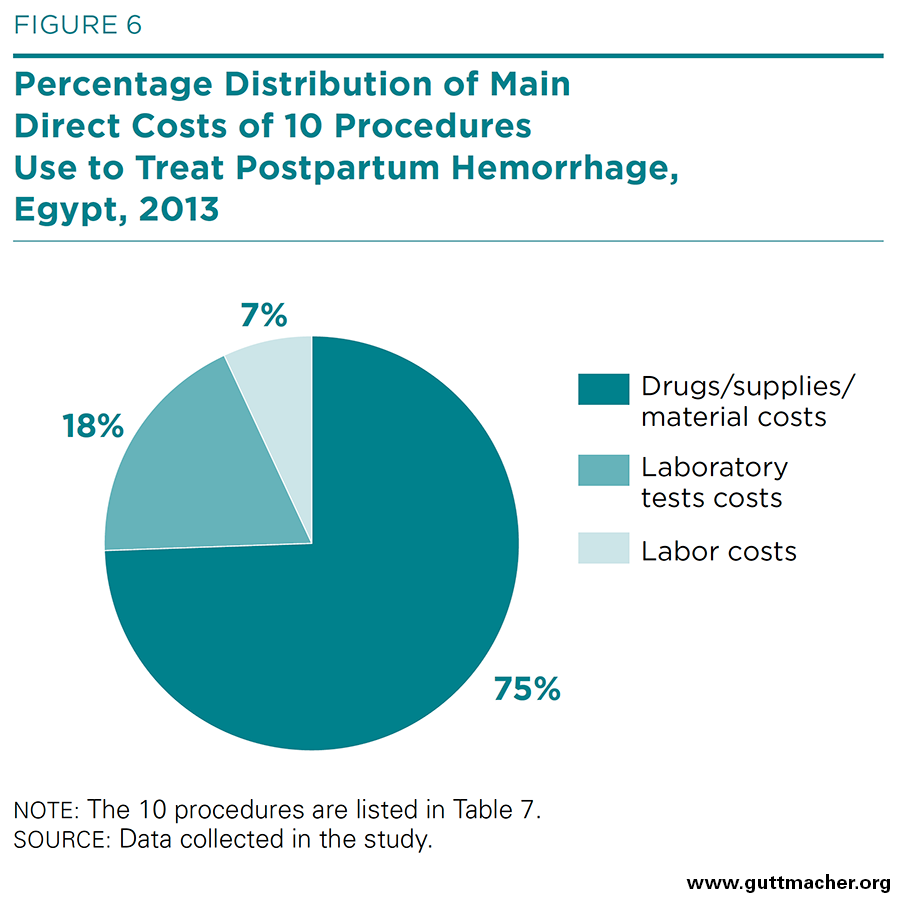
- The estimated total cost to treat one patient with PPH amounts to roughly 730 Egyptian pounds (£E), or US$105.00. Labor contributes 7% to this total; laboratory tests 18%; and drugs, supplies and materials (including blood products) 75%.
- This estimate refers only to inputs that go directly into medical treatment, namely, labor, physical items and laboratory tests. Indirect costs—those for infrastructure and overhead—may be equal to direct costs or even higher.
- For 2013, we estimate that the direct costs to the Egyptian health system were about £E20.5 million (US$3 million) to treat an estimated 28,000 cases of PPH. The actual total cost, including indirect costs, may be £E40 million (US$6 million) or more.
- The share of the Ministry of Health and Population budget dedicated to treatment of PPH is roughly 0.7% of its total.
- Given this comparatively moderate expenditure and the persistence of PPH as a public health problem having substantial morbidity and mortality, additional funds should be allocated to its prevention and treatment.
Introduction
Obstetric hemorrhage is the leading cause of maternal mortality* in developing countries, accounting for 27% of all maternal deaths that occur worldwide. Of the three types of hemorrhage—antepartum, intrapartum and postpartum—the postpartum type is by far the most important, accounting for 73% of all hemorrhage cases.1 Deaths from postpartum hemorrhage (PPH) are concentrated in developing countries, and slightly more than 43% of them occur in Northern and Sub-Saharan Africa. In Egypt, the maternal mortality ratio stood at 45 deaths per 100,000 live births in 2013 according to the World Health Organization (WHO).2 Although this ratio is not high by standards for developing countries, where the average is 230, it is still considerably above the average of 16 in developed countries.
PPH is formally defined as the loss of at least 500 ml of blood after a vaginal birth or the loss of at least 1,000 ml of blood after a cesarean section within 24 hours of delivery.3 WHO recommends various lines of treatment for PPH, including the use of uterotonics, agents that induce contraction or greater tone of the uterus.4 Administration of the uterotonic oxytocin intravenously is the medically preferred first line of treatment, but various attributes of the drug, particularly the need for refrigeration and trained personnel, make it difficult to maintain in low-resource settings. Where oxytocin is not available or cannot be properly stored, misoprostol, another WHO-recommended uterotonic that is administered sublingually, can be used as an alternative treatment.† 4,5
Maternal mortality declined substantially in Egypt during 1995–2008, from 230 to 66 deaths per 100,000 live births, according to estimates from successive Demographic and Health Surveys.6,7 Two national maternal mortality surveys, conducted in 1992–1993 and in 2000–2001, both found that about 30% of all maternal deaths were caused by PPH.8 Another study on maternal mortality in Egypt found that the main reasons for avoidable deaths were substandard care, delay in recognizing the condition, poor antenatal care and a lack of supplies including blood.9
For the last decade and a half, the Ministry of Health and Population has published yearly estimates of maternal mortality ratios in Egypt.10 According to this source, the maternal mortality ratio declined from 84 to 52 maternal deaths per 100,000 live births between 2000 and 2013 as shown in Figure 1.‡
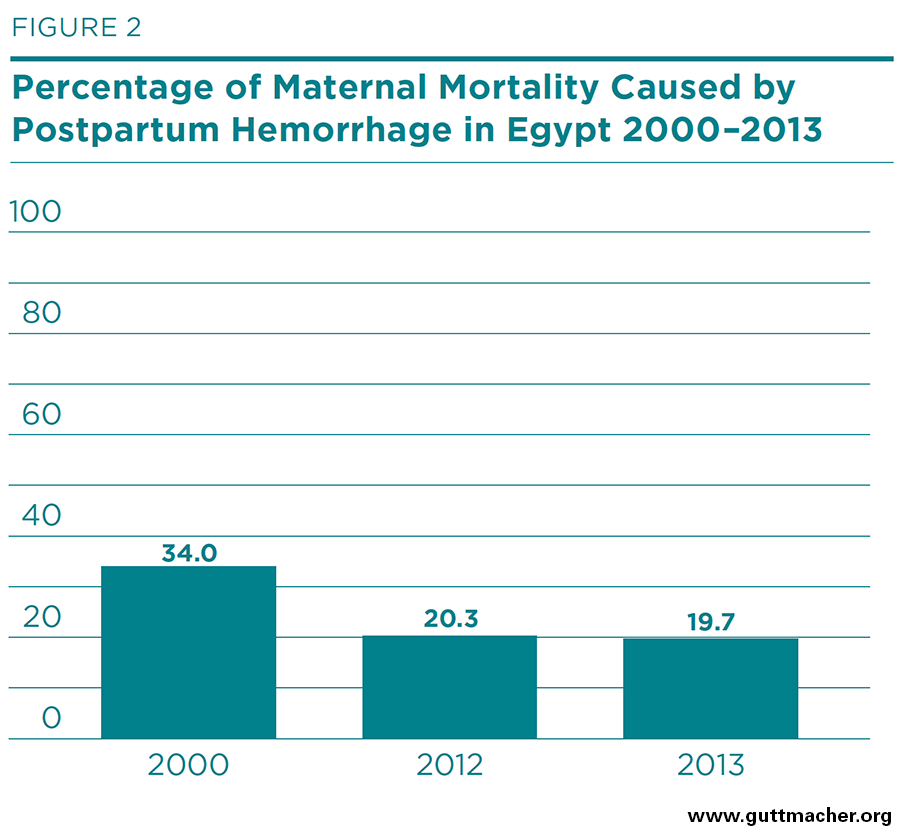
This ministry also publishes annual estimates of the direct causes of maternal deaths.§ Data from this source suggest that the percentage of maternal deaths attributable to PPH declined from 34% in 2000 to 20% in 2013 as shown in Figure 2.
The Ministry of Health and Population has spearheaded efforts to reduce maternal mortality and PPH as a cause of maternal deaths. This national priority includes focusing on regions where rates of maternal death and injury are highest (e.g., the Upper Egypt region), expanding health services to increase access to skilled routine and emergency obstetric care, and implementing a prenatal surveillance program, which helps to monitor the quality and frequency of antenatal care visits.11
In 2014, 87% of births in Egypt occurred in health facilities: 61% in private ones and 26% in public ones. The remaining 13% of births took place at home. With regard to assistance during delivery, 92% of women were attended by a doctor or trained nurse-midwife. Nearly all of the rest were attended by a daya, a traditional birth attendant.12 Egyptian health regulations prohibit the use of uterotonics in nonhospital settings, so women experiencing PPH during delivery at primary care sites, at private clinics or at home need to be transported to a hospital when the condition is diagnosed, increasing the health risk because of the delays in transportation involved.
During 2013–2014, Gynuity Health Projects and the Egyptian Ministry of Health and Population undertook a trial testing administration of misoprostol in community-level settings, specifically for deliveries at home and at primary care centers.** The aim of the trial was to assess the feasibility and effectiveness of adding a dose of 800 mcg sublingual misoprostol to standard of care (referral to a higher-level facility) among women with PPH in a variety of rural settings. The two primary research questions were whether administration of misoprostol as the first line of treatment for PPH at lower levels of health care—at primary care centers or at home by home birth staff—would reduce the proportion of women needing referral and the number experiencing major drops in hemoglobin levels (reductions of more than 2 g/dl) compared with standard of care alone.
A companion cost-effectiveness analysis was planned to follow the main study, with the intention of comparing the two strategies (misoprostol plus referral versus referral only) for the treatment of PPH in the population and settings studied. Specifically, the costs of the two interventions to the Egyptian health care system were to be estimated and their cost-effectiveness computed.
However, the results of the main study proved to be inconclusive: The combination of misoprostol and referral did not produce outcomes significantly different from those seen with referral alone. The original plan to do a cost-effectiveness analysis was therefore abandoned. In preparation for this analysis, however, investigation had been undertaken of the costs to the Egyptian health system of treating PPH cases referred to the secondary level of health care (i.e., midlevel/district hospitals). This report describes that costing analysis and presents its findings. As there is no literature on the cost of PPH in Egypt, this analysis fills an important knowledge gap with information that should prove valuable to policymakers and program planners both in Egypt and in the wider international health policy community.
Data Sources and Methods
Data for this costing analysis were collected through three main channels. First, we added questions to the data collection forms used in the main study of misoprostol. These questions asked about inputs, of both labor and physical items such as drugs, that were related to treating PPH once it had been diagnosed. Second, we collected data from the public health system at different levels through additional questionnaires. These data consisted of salary schedules as well as prices of blood products and laboratory tests. Third, in 2013, we polled a panel of experts consisting of administrators, doctors and other staff at the referral facility level and others conversant with the treatment of PPH in Egypt. Their responses constitute the majority of the costing data used for this report. (See Appendix Table 6, for more details on data sources.)
Expert Panel
The expert panel was convened to estimate the parameters needed to calculate per-case costs of treating PPH to the Egyptian health care system. We used information from the panel to estimate the cost of treating patients who were referred, transferred or both to district hospitals. Panel interviews were carried out under the guidance of a local health economist. The panel used a specially developed questionnaire (see Appendix B), through which the experts estimated various percentages, rates and costs based on their experience with treating PPH and their work at different levels of the Egyptian health care system. They were asked questions regarding the time spent by medical personnel on various procedures that are used in patients with PPH. The experts also provided information on patterns and standards of treatment, and answered questions on physical items used in the various procedures, including drugs, supplies and other materials.
Initially, we had planned a Delphi process13 with two rounds. The first round collected data from participants divided into two groups, each having 7–8 experts. The proposed second round was to be a consensus meeting, in which all experts were to discuss and adjust their answers to reach consensus on the quantitative aspects of the data. Unfortunately, this round had to be cancelled because of the political instability in Egypt during the study period.
The questionnaire was prepared in both the English and Arabic languages. It was pretested by doctors at Alexandria University Hospital (Shatby Hospital) in Alexandria and by a panel of junior staff in the Ob/Gyn department at El Galaa Maternity Hospital in Cairo. These pretesting activities led to some improvements in the questionnaire.
Medical professionals were recruited for the expert panel based on their level of experience (more than 10 years) and their expertise in treating PPH. They were selected from Etay El-Barood Hospital and Kafr El-Dwar Hospital in the El Beheira governorate, located in the northern part of the country, where the field work of the main study of misoprostol was based. Two panel sessions were held, one at each hospital. The panel from Etay El-Barood Hospital was composed of five physicians and two nurses; the panel from Kafr El-Dwar Hospital was composed of four physicians and four nurses. The experts included heads of Ob/Gyn departments, Ob/Gyn specialists, head nurses, supervising nurses and medical residents.
Each of the two panel sessions began with an explanation of the objective of the panel, a review of the questions on the questionnaire, and directions on how to fill it out. The experts then answered the questions under the supervision of the local health economist. On average, they spent about two hours completing the questionnaire. All information given by individual panelists was kept strictly confidential, and only group averages are reported. Each panelist received a reasonable honorarium for participation, and no panel interview exceeded four hours in length.
We designed a data entry sheet using the Excel software package (Microsoft). Data from the questionnaires were entered into the sheet twice: once by an obstetrician-gynecologist familiar with technical terms and abbreviations used in that area of medicine, and once by a member of the study team. The data were verified and errors were accordingly corrected. When there was ambiguity in the experts’ responses in terms of the formulations of a drug (e.g., tablet, capsule, syrup), we calculated average prices of the various possible formulations.
Cost Data
We collected data on the international prices of drugs, materials and supplies from various sources. These included the 2012 International Drug Price Indicator Guide,14 the International Dispensary Association Foundation 2012 e-Catalogue,15 the Ugandan Joint Medical Store 2012 Catalogue,16 the Durbin Medical Supply 2013 Catalogue,17 and the Cameroon National Association for Family Welfare (CAMNAFAW) 2013 Catalogue.18
National drug and material prices were available both as market prices and as subsidized prices. The market prices were ascertained from the Al Dawaa El Masry Handbook of Egyptian Drugs published in 2013, which contained a list of all drugs available on the Egyptian market. The subsidized prices were obtained from administrative documents at Etay El-Barood and Kafr El-Dwar Hospitals.†† We opted to use market prices and preferred local ones over international ones whenever the former were available. See the appendix tables in Appendix D for more detailed information on the drugs, supplies and materials used in treating PPH and any adjustments made to prices.
The costs of laboratory tests used in treating PPH were obtained from a survey of nine private laboratories, one in Cairo and eight in the El Beheira governorate, where data collection for the main study took place. We selected market prices from private establishments as these include all costs associated with testing. To be consistent with the other input cost data, which measured only direct costs, we adjusted the laboratory price data downward to exclude the indirect cost component.‡‡
We used administrative records and salary schedules to collect data on the labor inputs of seven cadres of medical professionals involved in treating PPH cases at midlevel hospitals: obstetrician-gynecologists, anesthesiologists, general practitioners, medical residents, house officers, nurses and auxiliary nurses.§§
Before the expert panel met, a four-member panel consisting of prominent medical practitioners was convened to create a typology of medical procedures used in the treatment of PPH in Egypt (at all levels of care). The typology was developed by consensus and consists of 24 procedures.*† The procedures range from simple manual ones, such as uterine massage, and cord clamping and controlled cord traction, to complex surgical ones. They also include administration of drugs (mainly uterotonics, but sedatives or anesthetics as well) and transfusions.
We conducted a number of literature searches using the PubMed database to identify possible studies of PPH costs in Egypt and elsewhere. We searched the database using the MeSH (Medical Subject Headings)*‡ terms "hemorrhage, postpartum" and "misoprostol," and narrowed the publication dates to the period 2005–2015. This search yielded 182 publications. The same method was followed using the MeSH terms "oxytocin" and "hemorrhage, postpartum" (206 results) and "oxytocin" and "hemorrhage, postpartum" and "treatment" (17 results). Furthermore, we searched the International Journal of Gynecology & Obstetrics using the terms "misoprostol" and "treatment" and "postpartum hemorrhage," finding 327 results using a date range of 2005–2015. Additional literature was identified by perusing the reference lists of articles already found from the above-mentioned search results.
Computations
We computed the per-case costs of the various inputs with straightforward accounting-type calculations. Computation of the cost of labor can be summarized by the following three equations, in which a cadre refers to a given category of medical professional.
In this study, we look at only direct costs—the labor and physical inputs (such as drugs)—required to treat PPH cases.†* Of almost equal importance are indirect costs—all of the health system expenses that support the provision of health care even though they do not play a direct role in treatment. These indirect costs are generally capital and overhead costs, and include, for example, administrative, infrastructure and operating costs. The cost of hospitalization is mostly made up of indirect costs.
Key Findings
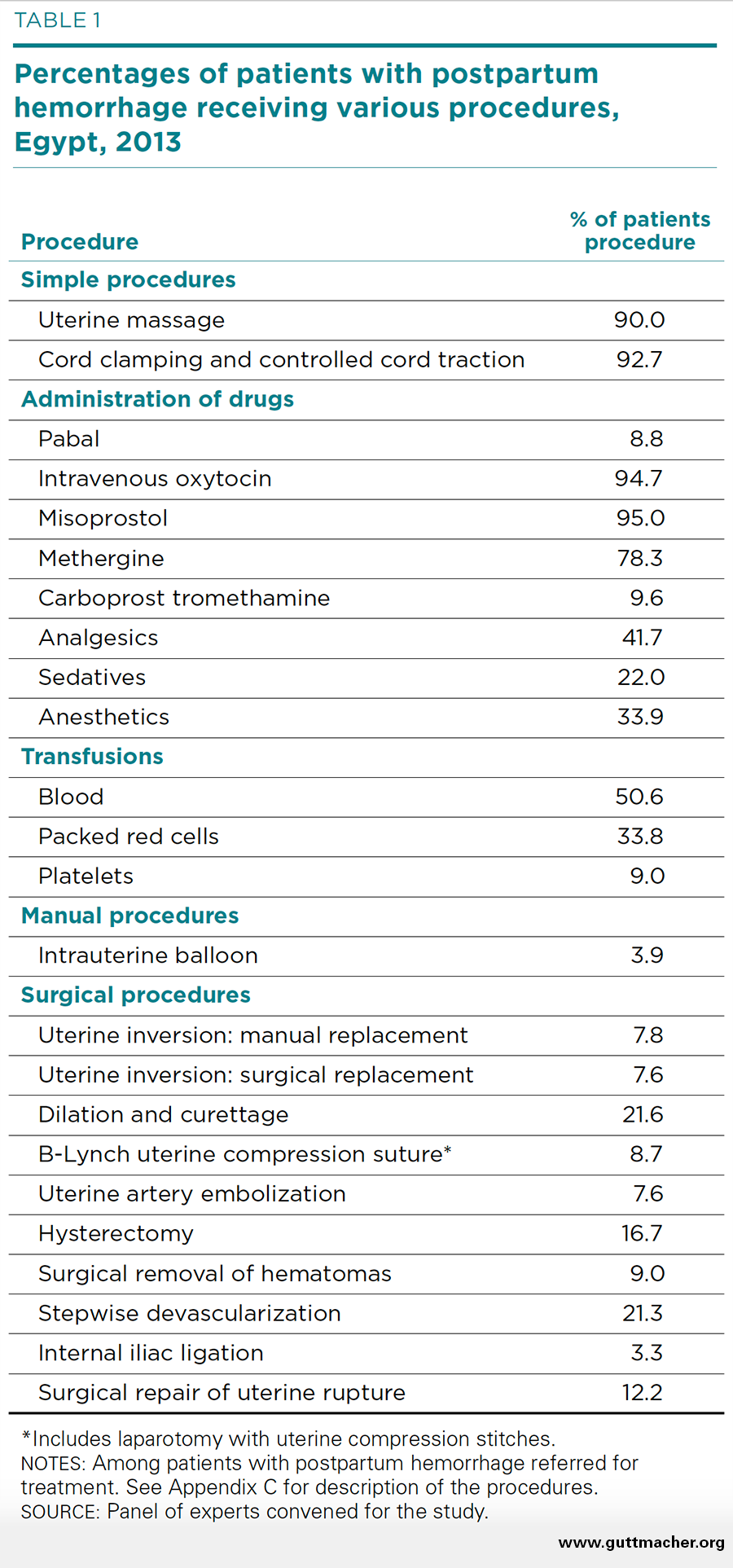
The expert panel estimated the percentage of patients referred for treatment of PPH who receive each of the 24 procedures. These percentages therefore represent estimates of the typical care provided to such patients at midlevel hospitals such as Etay El-Barood Hospital and Kafr El-Dwar Hospital in El Beheira governorate, where the main study was undertaken.
Almost all patients referred for PPH (90–93%) receive the simple manual procedures of massage and controlled cord traction (Table 1). Transfusion of blood or a blood product is also nearly universal (93%). The use of multiple uterotonics is very common as well: The vast majority of patients receive both oxytocin and misoprostol (95% for each). The leading surgical procedures performed are dilation and curettage (22%) and stepwise devascularization (21%). Hysterectomy and repair of uterine rupture are also fairly common (17% and 12%, respectively).
Labor Costs
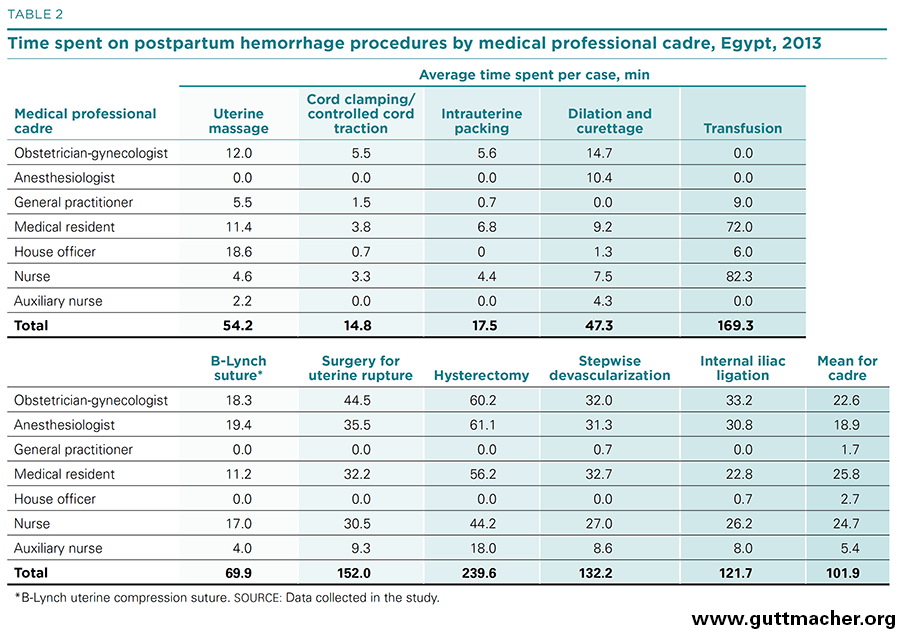
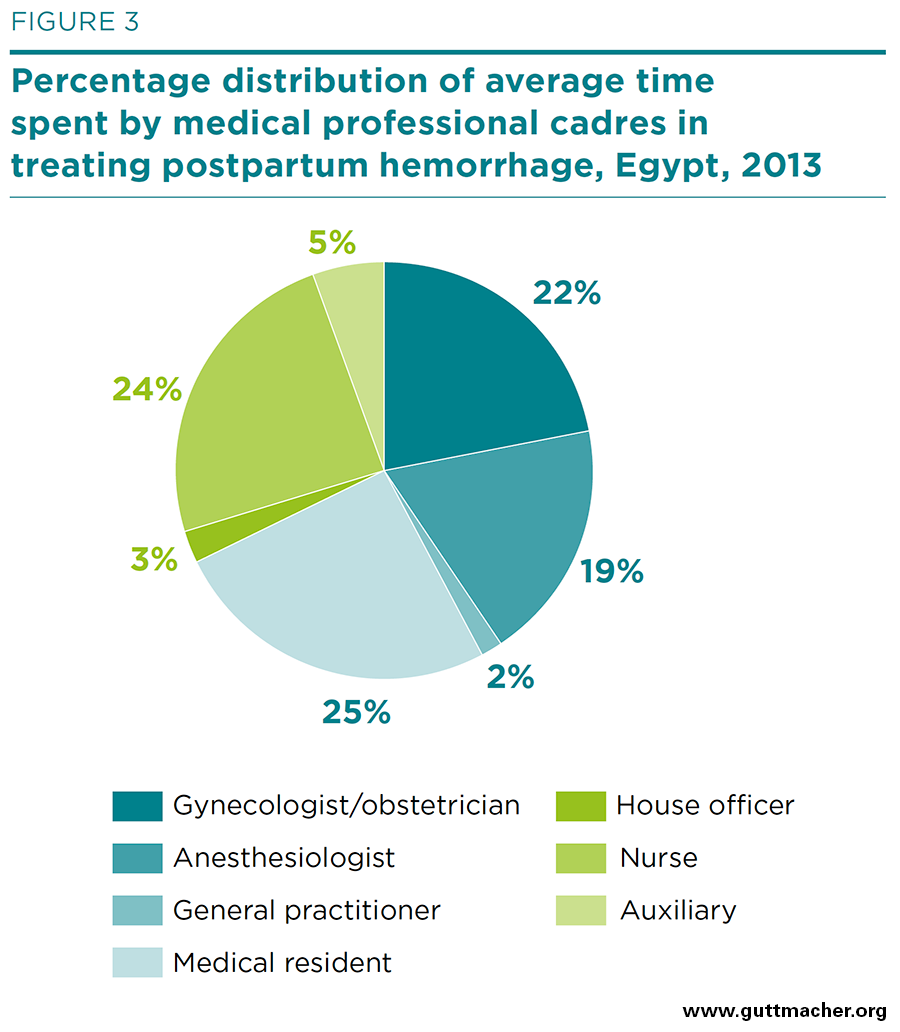
As described above, labor costs for treating PPH in referred patients were disaggregated by both medical professional cadre and medical procedure. The amount of time spent by various medical professionals for 10 procedures is shown in Table 2, and the percentage distribution by cadre is shown in Figure 3. Nurses and medical residents spend the most time treating patients with PPH (25–26 minutes, on average), followed by obstetrician-gynecologists and anesthesiologists (19–23 minutes). General practitioners and house officers have little involvement (2–3 minutes).
Medical professionals spend more time performing hysterectomies (240 person-minutes in total per operation) than any other PPH procedure. Moreover, about half of the total personnel time is spent by the most trained professionals. Transfusions, although requiring the second largest input of skilled labor (169 minutes), rely more heavily on professionals having less training. Internal iliac ligation, repair of uterine rupture and stepwise devascularization are also labor-intensive PPH procedures (taking 122–152 minutes each). On the other hand, intrauterine packing, and cord clamping and controlled cord traction require relatively minimal time (15–18 minutes).
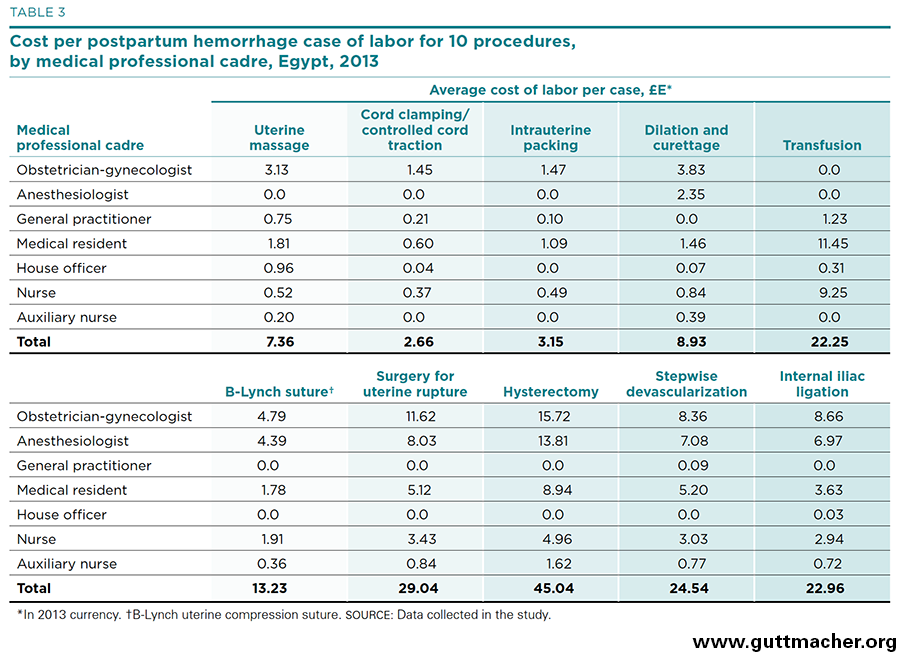
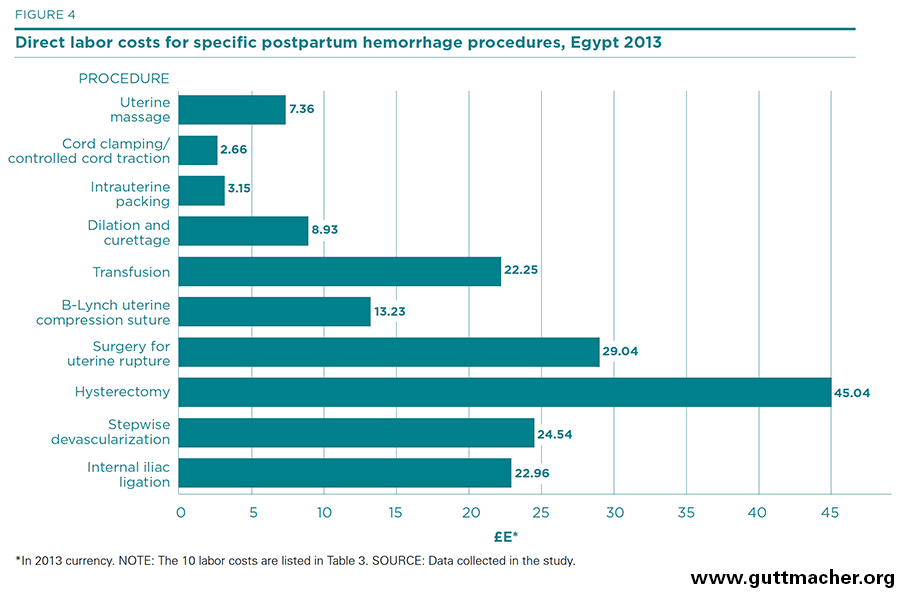
Table 3 and Figure 4 present direct labor costs per case for each of 10 procedures used to treat PPH, measured in 2013 Egyptian pounds (£E).†‡ The costs range from about £E2.70 (for cord clamping and controlled cord traction) to £E45.00 (for hysterectomy). In U.S. dollars, they range from US$0.38 to US$6.49. All in all, the cost of labor inputs can be categorized as moderate by international standards. In reality, these estimates likely underestimate the true cost of medical personnel as they may not adequately account for time when the salaried workers are not treating cases, even though the expert panel was asked to estimate the nontreatment time of the various medical cadres (see Appendix Table 2). Salary estimates (Appendix Table 1) may also underestimate the true cost of labor, which may include several benefits and perquisites besides monetary payments.
Physical Input Costs
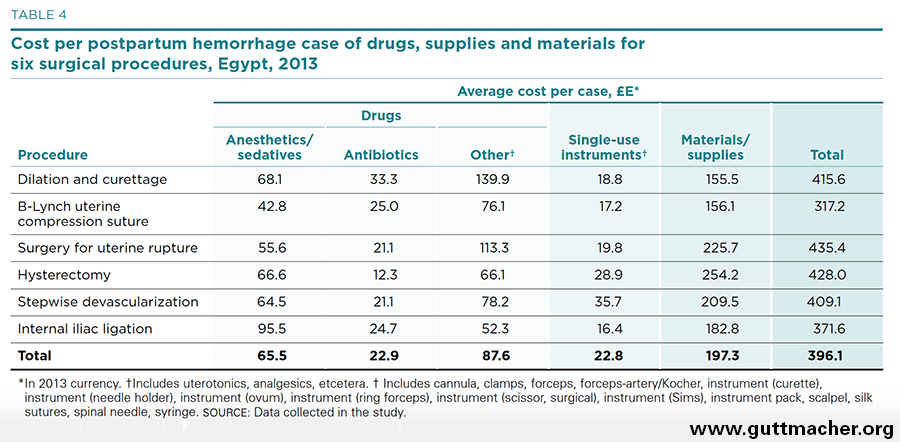
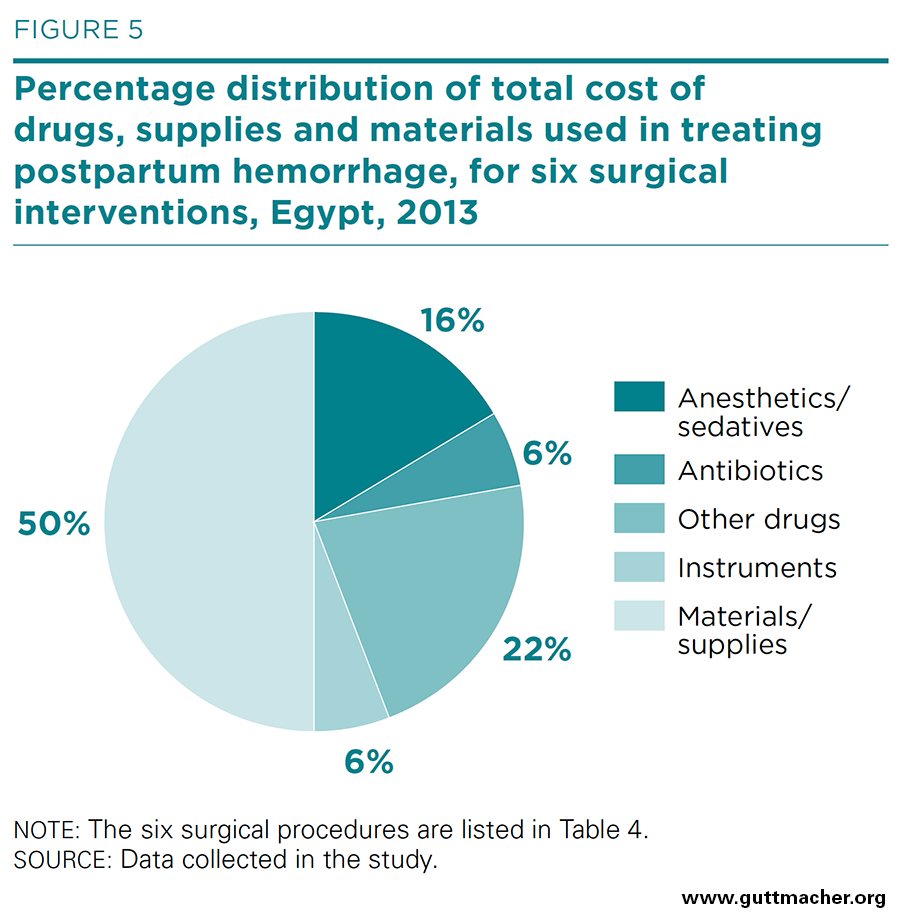
The average costs of drugs, supplies and materials used in the six major surgical procedures for PPH are presented in Table 4, and the percent distribution is shown in Figure 5. Although the costs of labor inputs were moderate, the costs of drugs and other supplies were high, ranging from £E317 (US$45.70) for placement of a B-Lynch uterine compression suture to £E435 (US$62.70) for surgery to repair uterine rupture. In total, treating the average case of PPH required the expenditure of £E396 (US$57.10) for drugs, supplies and materials. Drugs for sedation or anesthesia were a major contributor to this cost, accounting for 17% of the average total cost of physical inputs (Appendix Table 5). Single-use instruments, on the other hand, contributed little to this total, just £E22.8 (US$3.30).
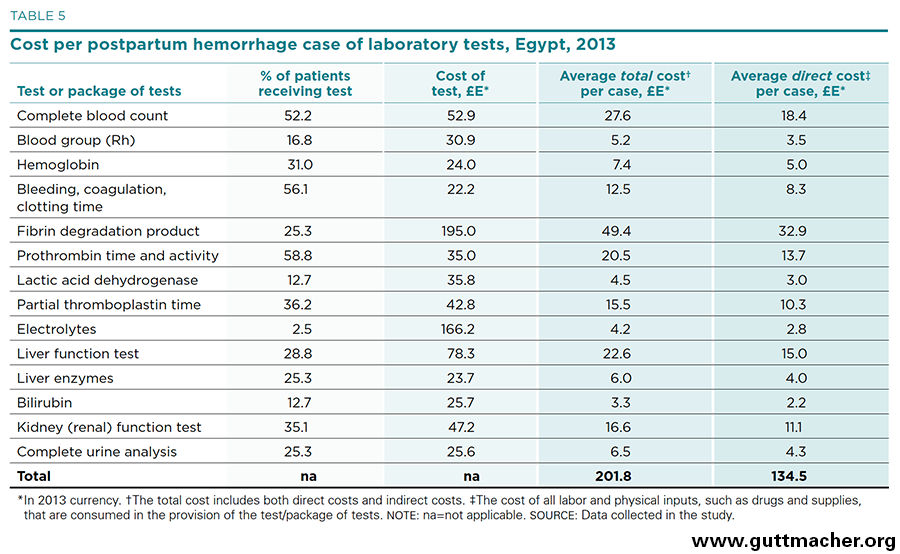
Another important driver of PPH treatment cost is the battery of laboratory tests frequently ordered for patients. Table 5 shows the percentage of patients for whom various tests were ordered, the cost of each test, the average total costs of such tests per case (i.e., including both direct and indirect components) and the average direct costs of each test per case. Three of the tests are ordered in more than 50% of patients with PPH, and seven are ordered in 25–50%. On average, each patient receives more than four tests. The cost of the tests themselves averages roughly £E58 (US$8.30), but because multiple tests are used in the typical patient with PPH, the total direct cost for the health system is about £E135 (US$19.40) per patient.†§ The single most expensive laboratory test, from the health system perspective, is the fibrin degradation product test, not only because of its high cost (£E195), but also because it is used in more than one-quarter of all patients with PPH.
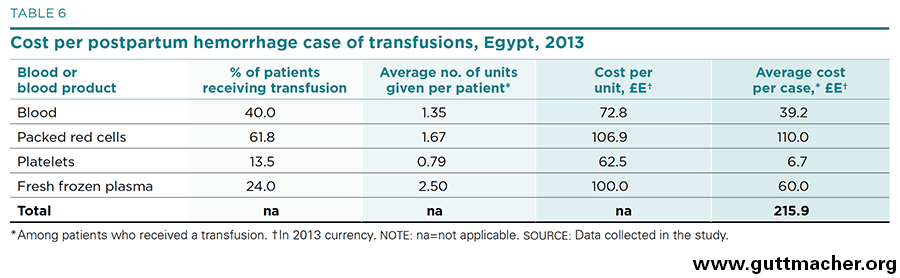
The cost of transfusions of blood or blood products merits special attention because it is generally an important driver of total cost and also because the price of blood is often influenced by special country-specific considerations. In Egypt, blood is regularly supplied by family members when the need arises, but is also provided by vendors on a commercial basis. Table 6 gives the commercial costs based on prices estimated by the expert panel. Of those patients with PPH who are given transfusions, 62% receive packed red cells and 40% receive whole blood.‡* Many patients are given more than one blood product and, on average, more than six units of blood or a blood product are transfused. Packed red cells are the most costly, at £E107 (US$15.42) per unit, as well as being the most commonly used blood product. Thus, they contribute the greatest share—51% or £E110 (US$15.85)—to the average cost of transfusions per patient with PPH given this treatment, which totals to about £E216 (US$31.10).
Total Cost per Case
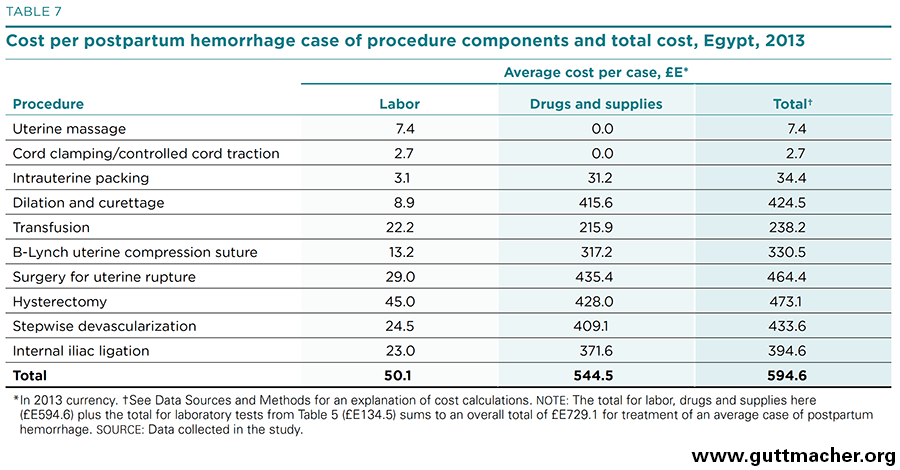
Table 7 aggregates the direct cost components of treating PPH in midlevel hospitals based on the findings for the individual cost components. For the 10 procedures listed in the table, the labor component costs £E50 (US$7.20) per average PPH case, and the sum of physical inputs costs £E545 (US$78.50). When laboratory tests are added, the estimated total average cost to treat one patient with PPH amounts to roughly £E730 (US$105.00). In proportional terms, as shown in Figure 6, labor contributes 7% to the total cost per case; laboratory tests 18%; and drugs, supplies and materials (including blood products) 75%. Note that these total cost estimates may be underestimated because the labor component for a few infrequently used procedures was not included.‡†
Discussion
The main objective of this study was to estimate the cost to the Egyptian health system of treating PPH cases in two rural districts. Keeping in mind that the data used come from a single governorate, we estimated the average cost per case, that is, per patient referred for PPH, to be £E730 (US$105) in 2013. This estimate refers to only the inputs that go directly into the medical treatment of a PPH patient, namely, labor and physical items such as drugs, tests, supplies and materials. Other costing studies have shown that indirect costs—infrastructure and overhead costs—may be equal to direct costs or even higher.‡§ In other words, the total cost to the health system could be double the direct cost estimated here, or even more.§* It should also be noted that the perspective chosen in this study was that of the health system. Had we chosen another perspective, for example, a societal one, we would have had to add to the analysis many other costs, such as opportunity costs, loss-of-productivity costs and intergenerational costs.§†
We recognize several limitations in the design of this study. Perhaps the most important one is that the aim of the research changed once the main study’s results could not detect effects from the misoprostol intervention. Our plan to undertake a cost-effectiveness analysis became untenable, and a new objective was adopted, namely, to produce a descriptive report on the cost of PPH to the Egyptian health system. This change of tack meant that the type of data collected and the method of collection were not ideal. For instance, although the expert panel approach was an economical one considered adequate for a cost-effectiveness analysis, a more robust data-gathering method would have been preferred for research aimed solely at cost estimation. Likewise, our study did not collect data on indirect costs. Had the focus been narrowly on PPH costs, data on indirect costs would have been collected. Furthermore, the primary data of our study were collected in only one district of Egypt, so generalizing our findings to the whole country is risky. On the other hand, the component approach we adopted likely minimizes this risk because the unit costs of the various inputs may not vary much from one part of Egypt to another. Finally, in our calculations of the total annual cost of treating PPH, we had to make the assumption that the number of cases of PPH referred equals the number of cases of severe PPH, for which only one estimate is available.21 Unfortunately, there are no data showing what proportion of severe cases are diagnosed and receive treatment at referral centers.
Given the per-case estimate of treatment costs, we are able to make a rough estimate of what the total national annual cost to the health system of caring for all cases of PPH referred to a hospital. For 2013, we estimate that it cost about £E20.5 million (US$3 million) to treat the estimated 28,000 cases of PPH that occurred in Egypt.§‡ 21,22 Again, this amount refers only to the direct costs of treatment. The actual total cost, including indirect costs, might rise to £E40 million (US$6 million) or more. It should also be noted that this total national cost assumes that all women needing care at referral hospitals are able to access such care. To the extent that poor or rural women may not have access to care in health facilities because of cost or distance, this total represents an estimate of system costs under the ideal condition of total access to health care rather than an estimate of the costs currently accrued.
To put our findings into context, for 2007–2008, the total health expenditure in Egypt was estimated to be £E42.5 billion, and spending by the Ministry of Health and Population was estimated to be £E10.2 billion.23 Assuming that the total expenditure on PPH is as high as £E40 million, the share dedicated to PPH accounts for less than one-half percent of the total expenditure on health in Egypt and roughly 0.7% of the Ministry of Health and Population total annual budget. As the amount of money spent on treating PPH is moderate compared with the total health bill in Egypt, and this condition remains a persistent public health problem causing substantial morbidity and mortality, additional funds should be allocated to the area of PPH prevention and treatment. One way in which extra funding could be used would be to strengthen support systems and to ensure that all women at the time of childbirth are able to reach an appropriate health facility should postpartum bleeding become excessive and require urgent and prompt care. Alternatively, more money could be allocated to PPH prevention and treatment by improving management of the condition both at the community level and in hospital settings, including access to and appropriate use of uterotonics.***
At a global level, this study, even though based on data from only a single district of Egypt, is the first to attempt to estimate the cost of treating referred cases of PPH. Thus, the costs estimated here are of interest to the wider health economics community in that they are based on data and so can be used as a reality check in other settings that thus far lack empirical evidence.
References
1. Say L et al., Global causes of maternal death: a WHO systematic analysis, Lancet Global Health, 2014, 2(6):e323-e333.
2. WHO and World Bank, Trends in Maternal Mortality: 1990 to 2013. Estimates by WHO, UNICEF, UNFPA, Geneva: WHO, 2014.
3. ACOG, Postpartum hemorrhage, ACOG Practice Bulletin, Clinical Guidelines for Obstetrician-Gynecologists,2006, No. 76.
4. WHO, WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage, Geneva: WHO, 2012.
5. Hofmeyr GJ et al., Misoprostol to prevent and treat postpartum haemorrhage: a systematic review and meta-analysis of maternal deaths and dose-related effects, Bulletin of the World Health Organization, 2009, 87(9):666-677.
6. El-Zanaty F, Hussein EM and Shawky GA, Egypt Demographic and Health Survey 1995, Calverton, Maryland: National Population Council [Egypt] and Macro International Inc., 1996.
7. El-Zanaty F and Way A, Egypt Demographic and Health Survey 2008, Cairo, Egypt: Ministry of Health, El-Zanaty, and Associates and Macro International, 2009.
8. Amin TT, Maternal and perinatal mortality: a snapshot on the Egyptian situation, International Public Health Forum, 2014, 1(3):1-5.
9. Campbell O et al., National maternal mortality ratio in Egypt halved between 1992-93 and 2000, Bulletin of the World Health Organization, 2005, 83(6):462-471.
10. Ministry of Health and Population, Directorate of Maternal and Child Health Care, National Maternal Mortality Study, 2000, Cairo, Egypt: Ministry of Health and Population, Directorate of Maternal and Child Health Care, 2001.
11. Women Deliver, Reducing Maternal Mortality: Countries to Inspire Continued Commitment, 2011, http://www.womendeliver.org/assets/Maternal_Mortality_Success_Stories.p….
12. Ministry of Health and Populaiton [Egypt], El-Zanaty and Associates [Egypt] and ICF International, Egypt Demographic and Health Survey 2014, Cairo, Egypt and Rockville, Maryland, USA: Ministry of Health and Population and ICF International, 2015.
13. Brown BB, Delphi Process: A Methodology Used for the Elicitation of Opinions of Experts, Santa Monica, CA: RAND Corporation, 1968.
14. Management Sciences for Health, International Drug Prices Indicator Guide, 2012 Edition, Arlington, VA: Management Sciences for Health, 2013.
15. International Dispensary Association Foundation, International Dispensary Association's E-Catalogue July 2012, Amsterdam: International Dispensary Association Foundation, 2012.
16. Joint Medical Store, Joint Medical Store 2012 Catalogue, Kampala, Uganda: Joint Medical Store, 2012.
17. Durbin PLC, Durbin Medical Supply 2013 Catalogue, Middlesex, UK: Durbin PLC, 2012.
18. Cameroon National Association for Family Welfare (CAMNAFAW, Cameroon National Association for Family Welfare 2013 Catalogue, Yaoundé Cameroon: CAMNAFAW, 2012.
19. Vlassoff M et al., The health system cost of post-abortion care in Uganda, Health Policy and Planning, 2012, 29(1):56-66.
20. Vlassoff M et al., The health system cost of post-abortion care in Rwanda, Health Policy and Planning, 2014, 30(2):223-233.
21. Prata N et al., Inability to predict postpartum hemorrhage: insights from Egyptian intervention data, BMC Pregnancy and Childbirth, 2011, 11:97.
22. Carroli G et al., Epidemiology of postpartum haemorrhage: a systematic review, Best Practice & Research Clinical Obstetrics & Gynaecology, 2008, 22(6):999-1012.
23. Nakhimovsky S et al., Egypt National Health Accounts: 2008/09, Bethesda, MD: Health Systems 20/20 project, Abt Associates Inc., 2011.
Footnotes
*Death during pregnancy, childbirth or the postpartum period from any cause related to or aggravated by the pregnancy (source: World Health Organization, Maternal mortality, 2015, http://www.who.int/mediacentre/factsheets/fs348/en/).
†See Appendix A for more detail on treating PPH with misoprostol.
‡Note that the Ministry of Health and Population estimates differ slightly from the WHO estimates. The difference, however, is not significant given the problems surrounding the measurement of maternal mortality.
§Direct causes are those related to obstetric complications, such as hemorrhage, infection and hypertensive disorders; indirect causes are those related to preexisting conditions that are aggravated by pregnancy, such as anemia, malaria and HIV infection (source: Columbia University Mailman School of Public Health, Causes of maternal mortality, http://healthandrights.ccnmtl.columbia.edu/reproductive_health/causes_maternal_mortality.html).
**The results of the study have not yet been published.
††In addition, data on the salary and benefits of various cadres of medical professionals were obtained from administrative records at Etay El-Barood Hospital.
‡‡We assume that indirect costs are 50% of total costs. Hence, the adjustment factor is 0.67.
§§A medical resident is a health professional who has a bachelor of science degree in medicine and has spent a complete year as a trainee (as a house officer) in a tertiary hospital. The resident must have a specialty in a particular area of medicine. Residents serve 3–5 years in this position. A house officer is a health professional who has a bachelor of science degree in medicine and has spent a complete year as a trainee doing five "rotations," each two months long and in a different area of medicine
*†Uterine massage; cord clamping and controlled cord traction; administration of pabal, intravenous oxytocin, misoprostol, methergine, carboprost tromethamine, analgesics, sedatives and anesthetics; transfusion of blood, packed red cells and platelets; intrauterine balloon; manual replacement of uterine inversion; surgical replacement of uterine inversion; dilation and curettage; B-Lynch uterine compression suture; uterine artery embolization; hysterectomy; surgical removal of hematomas; stepwise devascularization; internal iliac ligation; and surgical repair of uterine rupture. A brief description of some of these interventions is given in Appendix C.
*‡MeSH is the National Library of Medicine's controlled vocabulary thesaurus. It consists of sets of terms naming descriptors in a hierarchical structure that permits searching at various levels of specificity.
*§The expert panel estimated the proportion of work time that medical personnel spent in activities other than treatment (e.g., administrative tasks, preparing the operating room, training activities, idle time). We anticipate that nontreatment time was underreported.
†*As the original aim of the study was to perform a cost-effectiveness analysis, we did not measure indirect costs under the assumption that they would be equal for both treatment interventions.
†‡The exchange rate between Egyptian pounds and U.S. dollars used throughout this report is £E6.94=US$1.00.
†§Note that these are direct costs. As information on the costs of laboratory tests was gathered from private entities, the costs include both direct and indirect components. To make these costs consistent with labor costs, we adjusted them downward by assuming that indirect costs were 50% of total costs.
‡*Note that the percentages presented in Table 6 have, as their denominator, those PPH patients who are given transfusions. By contrast, the corresponding percentages in Table 1 have as their denominators all PPH patients.
‡†These noncosted interventions are uterine inversion with manual replacement, uterine inversion with surgical replacement, uterine artery embolization and surgical removal of hematomas. These four interventions do not have cost estimates for labor inputs (but physical inputs were estimated). They are interventions that are comparatively rare, and we opted to omit them from the expert panel questionnaire to prevent it from becoming too lengthy and cumbersome. Another consideration favoring omission was that PPH covers only hemorrhage that is due to lack of uterine tone. These four interventions may, to a degree, be used to treat hemorrhage resulting from other conditions, such as trauma.
‡§See, for instance, Vlassoff et al. (2012, reference 19) and Vlassoff et al. (2014, reference 20).
§*Indirect costs were not collected in the study because the original objective, a cost-effectiveness analysis, did not require the calculation of such costs. We recognize this as a limitation of the study as indirect costs are a sizable proportion of total costs. Indirect costs included capital costs and overhead costs (administrative costs, utility and maintenance costs, training costs, etc.).
§†From the individual perspective, loss of income due to illness would be a relevant cost that should be counted. From a societal perspective, the loss of production during illness is a pertinent cost, as is the intergenerational cost if illness impinges on the nutritional, health or educational status of children resulting from maternal morbidity or mortality.
§‡The main assumptions that we made in order to calculate the total cost were as follows: (1) all cases of severe PPH (loss of 1,000 ml of blood or more) needed to be treated at a referral hospital and (2) the proportion of PPH cases that are severe cases is that given by Carroli et al. (reference 22), The estimate of PPH incidence in Egypt is taken from Prata N et al. (reference 21)
***This report did not aim to discuss the policy measures that would be most appropriate to respond to this need (which might include, inter alia, improved transportation, promotion of facility-based deliveries, interventions aimed at the prevention of PPH).
Suggested Citation
Vlassoff M, Abdalla HA and Gor V, The Cost to the Health System of Postpartum Hemorrhage in Egypt, New York: Guttmacher Institute, 2016.
Acknowledgments
This report was written by Michael Vlassoff and Vivian Gor, both of the Guttmacher Institute, and H. A. Abdalla, an independent consultant. It was edited by Susan London, an independent consultant.
The authors wish to thank the following people for their valuable contributions to this research: Dr. Emad Darwish, Shatby Hospital, University of Alexandria, Alexandria, Egypt; Dr. Mohamed Cherine Ramadan, El Galaa Maternity Hospital, Cairo; Dr. Neveen Hassanein, independent consultant, Cairo; Dr. Mohamed Nour, Ministry of Health and Population, Cairo; and Ms. Miral Breebaart, independent consultant, Cairo.
The study was funded by Gynuity Health Projects through a grant from the Bill & Melinda Gates Foundation. Additional support was provided by the Guttmacher Center for Population Research Innovation and Dissemination (NIH grant 5 R24 HD074034). The Guttmacher Institute also gratefully acknowledges the generous unrestricted support it receives from individuals and foundations—including major grants from The William and Flora Hewlett Foundation and the David and Lucile Packard Foundation—which undergirds all of the Institute’s work.
© Guttmacher Institute 2016
Additional Downloads
- Egypt PPH Appendix A: Misoprostol egypt-pph-appendix-a.pdf
- Egypt PPH Appendix B: Questionnaire egypt-pph-appendix-b.pdf
- Egypt PPH Appendix C: Description of Procedures egypt-pph-appendix-c.pdf
- Egypt PPH Appendix D: Tables egypt-pph-appendix-d-tables.pdf